If you or a loved one have taken (or affected by) Taxotere after January 1, 2005 please contact us today.
Taxotere Linked to Permanent Hair Loss In Breast Cancer Patients
If a diagnosis for breast cancer isn’t bad enough, imagine recovering from this deadly disease only to find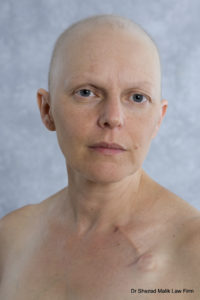 out that the drug used to treat the cancer has caused permanent hair loss. This is becoming a more common scenario for many breast cancer survivors, thanks to the popular chemotherapy drug, Taxotere, made by Sanofi-Aventis. Victims of Taxotere permanent alopecia can seek legal recourse by filing a Taxotere lawsuits. Our mass tort attorneys are currently investigating Taxotere lawsuits in all states and Washington D.C.
out that the drug used to treat the cancer has caused permanent hair loss. This is becoming a more common scenario for many breast cancer survivors, thanks to the popular chemotherapy drug, Taxotere, made by Sanofi-Aventis. Victims of Taxotere permanent alopecia can seek legal recourse by filing a Taxotere lawsuits. Our mass tort attorneys are currently investigating Taxotere lawsuits in all states and Washington D.C.

What Is Taxotere?
Originally approved by the FDA in 1996, Taxotere, also known as Docetaxel, is a synthetic chemotherapy drug used to treat a variety of cancers. Since 2005, Taxotere has become one of the most popular options for the treatment of breast cancer, with up to 75% of women receiving the drug as part of their treatment. Taxotere has been found to extend the survival rate for many breast cancer patients.
Taxotere works like many other chemotherapy drugs, stopping cell division and multiplication throughout the body. This damages cancer cells, preventing them from replicating and continuing to spread.
What Are The Risks Of Taking Taxotere?
While hair loss is a common side effect of many chemotherapy drugs, Taxotere has been linked to permanent hair loss, meaning that hair never grows back even after chemotherapy treatment is complete. Hair loss occurs all over the body including eyelashes, eyebrows and pubic hair. Sanofi-Aventis, the makers of Taxotere, funded a study which showed that 9.2% of patients suffered hair loss that lasted 10 years or longer.
Taxotere is sold in more than 90 countries. Sanofi-Aventis currently owns a 30-40% share of the market with an average of $3 billion in sales per year.
Prior to the introduction of Taxotere, Taxol, an all-natural chemical compound had been the drug of choice for breast cancer treatment. There have been no comprehensive studies showing that Taxotere and Docetaxel are more effective than Taxol.
What’s even worse is that the drug companies marketing Taxotere in the U.S. failed to warn U.S. consumers about the risks, despite the fact that warnings were given to European patients about the potential for permanent hair loss associated with Taxotere. Similar warnings were never issued in the United States.
The drug label was finally revised to include permanent “alopecia” on 12/11/15. See the FDA link for more information.